
So will claim these exemptions and circumvent all this to say it’s already approved and they can sell it, because identifying which category you fall into is a self-determination.” “They aren’t mixed with another product, and they don’t require the same demonstrations of safety and efficacy as manipulated stem cells might. “There is a set of regulations called tissue regulations which categorize certain products as minimally manipulated – meaning that they are used in the same manner in the recipient as they are in the donor,” said Dr. But they are still the patient’s own cells, and since they fall under a category classifying them as “minimally manipulated,” the FDA is limited in how much it can intervene. Unproven stem cell treatments typically follow the same pattern: cells are removed from a patient and then injected back into a different part of the patient’s body. And because of those types of exceptions, there’s sometimes some confusion.” “So for certain stem cell products, like when one is taking bone marrow from one individual and giving it to another as part of a stem cell transplant, there’s a very low level of regulation there. “The FDA looks to regulate things in the least burdensome way we can while also keeping products safe,” explained Dr.

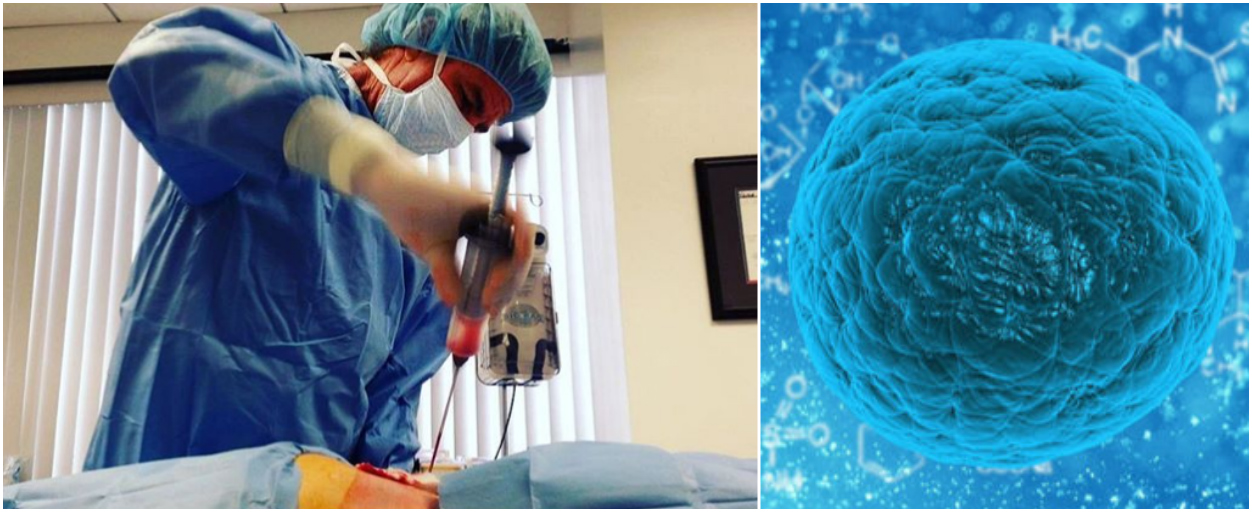
One reason the FDA has traditionally found it difficult to regulate these clinics is because it is unclear if the clinics fall within FDA oversight. How do predatory clinics bypass FDA regulations? These are the cells that are most commonly used and sold as an unlicensed product by some clinics,” noted Dr. “There is another population of adult stem cells called mesenchymal stem cells that are found in bone marrow, as well as in adipose tissue (fat), cord blood, and the placenta. However, some types of adult stem cells are being used in unproven therapies that can be harmful to patients: causing everything from tumors to blindness.

These are the stem cells used, for example, in lifesaving bone marrow transplants. Stem cells that exist in specific parts of the body and create a certain cell type are called adult stem cells. “For my work, I turn them into brain cells to study diseases like multiple sclerosis and Alzheimer’s, but they can be turned into virtually any type of cell in the body.” Stem-cell-derived astrocyte, a support cell in the brain. “In the past 15 years, scientists have learned how to take skin or blood samples and make what we call ‘ induced pluripotent stem cells ’ in a dish,” she said. Fossati’s research uses stem cells to study diseases of the brain. “For example, we can donate blood regularly because our blood is constantly replenished by a reservoir of stem cells that live in the bone marrow.”ĭr. “Our body has a remarkable regenerative capacity,” explained Dr. The two central features of stem cells are their ability to self-renew (create more of themselves) and become tissue or organ-specific cells with specialized functions. What are stem cells, and how do they work? Food and Drug Administration), Timothy Caulfield, LLM, FRSC, FCAHS (University of Alberta), Valentina Fossati, PhD (The NYSCF Research Institute), and Jeffrey Kahn, PhD, MPH (Johns Hopkins Berman Institute of Bioethics) to explore the science behind legitimate stem cell therapies and what these unproven stem cell clinics are offering patients. We recently convened experts Peter Marks, MD, PhD (U.S. “There are known and proven stem cell therapies on the market today, most significantly bone marrow transplants that have been transformational for many cancer patients however, the vast majority of stem cell treatments that are marketed for a huge breadth of devastating diseases are not only unproven – which means there’s no data to show that they work – but they also can be harmful to patients.” “In recent years, we have seen a huge proliferation of unproven stem cell therapies offered around the world and right here in the United States,” remarked NYSCF CEO Susan L. While many promising stem cell therapies are in development for heart disease, macular degeneration, Parkinson’s disease, and more, ‘bad actor’ clinics worldwide are exploiting the excitement around stem cells to sell expensive, unproven therapies to vulnerable patients with limited options. Off the side of the highway, between billboards for amusement parks and car dealerships, you may have encountered a new type of advertisement: “Cure your arthritis!” or “Get rid of your pain!” these billboards boast - “It’s all possible with the power of your own stem cells!”


 0 kommentar(er)
0 kommentar(er)
